Kesavardana Sannula

Kesavardana Sannula
Assistant Professor
Ph.D.: Indian Institute of Science, Bangalore, India.
Post-doctoral research:
St. Jude Children’s Research Hospital, Memphis, USA.
Year of Joining: 2020
Email: skesav@iisc.ac.in
Phone: +91 80 22932310
Highlights
- We study intracellular RNA sensing mechanisms and activation of functional higher-order complexes and how they orchestrate cell death, inflammationand virus-host interactions.
- We also explore the evolution of immune evasion traits in RNA viruses through a combination of protein engineering and structure-based rational approaches.
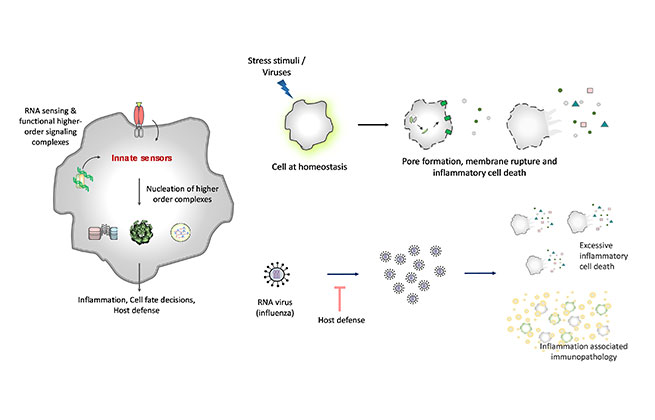
RNA sensing by innate nucleic acid sensors and assembly of functional higher-order signaling complexes regulating cellular functions; Activation of inflammatory cell death via membrane pore formation, membrane rupture and release of intracellular contents; RNA virus infection-induced immunopathology
RNA sensing :
Aberrant self-RNA conformations or viral RNAs act as intracellular threat indicators that are recognized by innate nucleic acid sensors. The activation of nucleic acid sensors trigger a variety of cellular functions and innate immune responses. Mutations in these nucleic acid sensors are associated with a variety of inflammatory diseases. We study RNA sensing mechanisms and activation of RNA sensors that specify antiviral immunity, inflammation, and cell death responses.
Inflammatory cell death :
The programmed activation of cell membrane permeabilization and rupture promotes lytic forms of cell death. This results in releasing intracellular contents, called damage-associated molecular patterns (DAMPs) and leaderless pro-inflammatory cytokines from dying cells. These DAMPs trigger inflammation in other cells promoting a cascade of inflammatory processes and thus this cell death is called ‘inflammatory cell death’
We study the mechanistic aspects of inflammatory cell deaths, necroptosis (driven by RHIM-proteins) and pyroptosis (triggered by Inflammasome) and hunt for novel pore-forming proteins and regulators of these processes. We also investigate the regulation of inflammatory cell death in response to RNA virus infections and viral strategies to evade cell death.
Functional higher-order complexes :
Eukaryotic cells have evolved to assemble higher-order oligomeric complexes, often called Functional Aggregates or Functional condensates, which appear as micron-scale intracellular compartments. The nucleation of these complexes is driven by 3D-folded domains, intrinsically disordered regions (IDRs), or by linear motifs. The formation of functional aggregates is an activation mechanism for several cellular functions, including nucleic acid sensing, stress adaptation, cell fate choices and inflammatory cell death. We study how these functional aggregates crosstalk for instructing cell fate decisions and how deregulation of this phenomenon prime viral pathogenesis and chronic human diseases.
Will be updated shortly
- Kesavardhana S et al., The Zα2 domain of ZBP1 is a molecular switch regulating influenza induced PANoptosis and perinatal lethality during development. J Biol Chem. 2020
- Kesavardhana S*, Malireddi RKS* et al., Caspases in cell death, inflammation and pyroptosis. Annu Rev Immunology.2020
- Samir P*, Kesavardhana S*, Patmore DM* et al., DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019
- Kesavardhana S et al., ZBP1/DAI ubiquitination and sensing of ribonucleoprotein complex activates programmed cell death. J Exp Med. 2017
- Man SM et al., IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016
- Karki R et al., NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature. 2016
Openings