Patrick D’Silva

DR. PATRICK D’ SILVA
Professor
Ph.D.: Indian Institute of Technology, Powai-Bombay
Post-doctoral research:
University of Wisconsin-Madison, USA
Year of Joining: 2007
Email: patrick@iisc.ac.in
Our lab research focusses on uncovering the pathways of mitochondrial protein translocation, biogenesis and turnover in connection to various pathophysiological conditions. By utilizing yeast and mammalian model systems, my lab will investigate the key regulatory mechanisms of redox homeostasis, protein trafficking & folding and organellar life cycle using a combination of experimental tools from genetics and cell biology with biochemistry.
Highlights
- Elucidated intricate organization & moonlighting functions of pre-sequence translocase
- Characterized therapeutic potential of nanozymes against oxidative stress disorders
Elucidating the organization of pre-sequence translocase and mechanisms of protein translocation across the mitochondria. Our studies delineate firsttime intricate organization of human pre-sequence translocase, which is significantly different from the yeast system. Our finding highlights the presence of 3 different translocase machinery for protein import across the mitochondrial inner membrane. Two of the translocases (B1 & B2) are essential for constitutive functions and translocase A found to be oncogenic in nature. Moreover, we uncovered that one of the components of the presequence machinery is involved in regulating apoptotic pathways thus imparting the chemoresistance in cancer cells. Our research findings provided first-time a direct connection between translocation machinery and the development of tumorigenicity. Our future long-term goal is to: (1) characterize the mitochondrial import motor and translocation machinery; (2) elucidate the mechanism and regulation of import motor component’s interactions with TIM23 channel to understand fundamental aspects of the mitochondrial protein transport process with respect to mitochondrial biogenesis in higher organisms; 3) characterize the organization of carrier translocase in connection to mitochondrial inner membrane protein biogenesis.
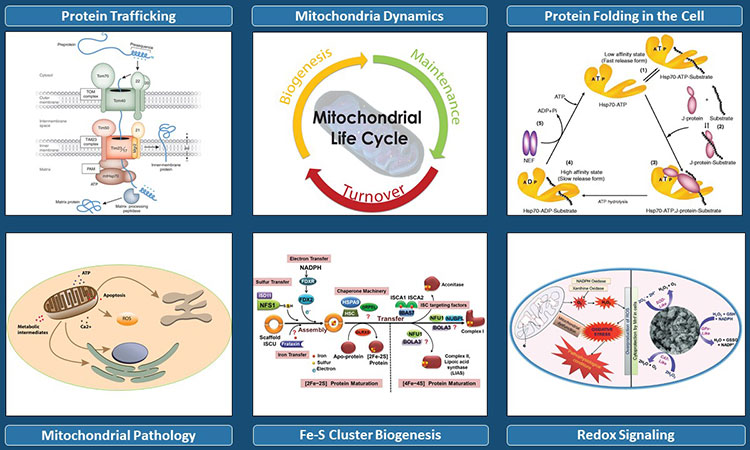
Regulation of mitochondrial dynamics by protein trafficking, iron-sulfur cluster biogenesis, protein folding in the cell and redox signaling.
Understanding Maintenance of mitochondrial quality control: Implications in human health. Mitochondria are the most dynamic and complex organelles that are involved in a wide range of cellular events, which are essential for tissue adaptation, survival, death, and renewal. Loss of mitochondrial protein quality control often leading organellar dysfunction is one of the important symptoms associated with Parkinson’s disease progression. Our research findings provide evidence in favor of mutations in folding machinery contributes to the progression of Parkinson’s disease. In addition to that, our findings highlight loss of protein quality control harboring mtHsp70 PD-variants leads to aggregation of α-synuclein and mitochondrial dysfunction. Our long-term goal would be to address how the organelle maintains its dynamics (fission-fusion cycles), membrane potential, and turnover (biogenesis and clearance) in normal and in pathophysiological conditions.
ROS signaling networks in eukaryotic systems:Cellular redox homeostasis is critically maintained by the equilibrium between ROS production and its removal through the involvement of well-defined antioxidant machinery. Any alteration in redox balance generates severe oxidative stress leading to multiple cellular damages. The association of ROS is well-known in several pathological conditions, including neurodegeneration, cancer progression, type two diabetes mellitus, and atherosclerosis. Our studies identify two novel classes of a ubiquitous redox-sensitive class of proteins (P16 and DJ-1) by genome-wide screen, which plays a critical role in ROS homeostasis in higher eukaryotes.Our future studies specifically focus on how DJ-1/Park7 class of proteins involved in ROS sensing and protection of organellar functions. The future study is also aimed to formulate potent biocompatible nanoparticles that can scavenge elevated ROS under pathological conditions.
Role of Heat Shock Proteins in Health and Diseases: The stressprotective heat-shock proteins are often overexpressed in cells of various cancers and have been suggested to be contributing factors in tumorigenesis. The overexpression of molecular chaperones also shown to protect cells against apoptotic cell death. Heat-shock proteins with dual roles as regulators of protein conformation and stress sensors may, therefore, have intriguing roles in both cell proliferation and apoptosis. The function of molecular chaperones is also vital for the aging process, autoimmunity, and the replication of many viruses. The involvement of chaperones, therefore, in such diverse roles suggests novel therapeutic approaches by targeting heat-shock protein function for a broad spectrum of tumor types, various pathogenic disease states, and protein conformational diseases.
Biogenesis of Iron-Sulphur Clusters (Fe/S centres) in Proteins: Fe/S clusters form an important moiety of proteins involved in a diverse cellular process. A vital function of mitochondria is the biogenesis of Fe/S clusters, as Fe/S cluster-containing proteins perform critical roles in cells, including electron transfer in oxidative phosphorylation. Mitochondrial molecular chaperones play an essential role in the synthesis and assembling of the Fe/S centres in most proteins that are targeted into different cellular locations.We will investigate the molecular mechanisms of Fe/S cluster formation and pathophysiological consequences due to impairment in the iron homeostasis using mammalian mitochondria.
- Uncovering the central role of human frataxin gene in cellular iron homeostasis; CSIR; 2012-2015
- Uncovering the role of human mitochondrial heat shock protein 70(mthsp70) in pathogenesis of Parkinson’s disease bad progression; LTMT- Young Researcher Award; 2012- 2017
- Uncovering multifunctional roles of mitochondrial heat shock proteins in neurodegenerative disorders and cancer progression; DST-Swarna Jayanti Fellowship; 2013-2018
- Exploring the therapeutic potential of V2O5 and Mn3O4 based Nanowires to prevent ROS-mediated cellular damages in a Parkinson’s disease (PD) mouse model system; DBT-National Bioscience Award; 2016-2019
- Uncovering the novel role(s) of Hsp31/DJ-1 family protein in the maintenance of mitochondrial health, DNA and protein homeostasis; DST-SERB, (2019-2021)
- Namrata Singh, Mohammed AzaruddinSavanur, Shubhi Srivastava, Patrick D’Silva,* Govindasamy Mugesh* (*Equal contribution). (2017), Redox Modulatory Mn3O4 Nanozyme with Multi-enzyme Activity Provides Efficient Cytoprotection to Human Cells in Parkinson’s Disease Model. Angew. Chem. Int. Ed., 6, 56(45), 14267-14271
- Matta SK, Pareek G, Bankapalli k, Anjaneya O and D’ Silva P. (2017) Role of Tim17 transmembrane regions in regulating the architecture of presequence translocase and mtDNA stability. Mol. Cell. Biol., 37(6), 491-16
- Vernekar AA, Sinha D, Srivastava S, Paramasivam PU, D’ Silva P*, Mugesh G* (*Equal Contribution). (2014) An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Communications, 21;5, 5301-06
- Samaddar M Goswami AV, Purushotham J, Hegde P, D’ Silva P. (Highlighted article by American Society of Cell Biology). (2014) Role of the loop L4,5 in allosteric regulation in mtHsp70s: in vivo significance of domain communication and its implications in protein translocation. Mol. Biol. Cell, 15, 25(14), 2129-42
- Goswami AV, Samaddar M, Sinha D, Purushotham J, D’ Silva P. (2012), Enhanced J-protein interaction and compromised protein stability of mtHsp70 variants lead to mitochondrial dysfunction in Parkinson’s disease. Hum. Mol. Genet., 21(15), 3317-32
Ph.D. Students: 10
Undergrads: 2
Postdocs: 0
Trainees: 1