Payel Roy

Payel Roy
Assistant Professor
Ph.D: Natioanl Institute of Immunology, New Delhi, India
Post-doctoral research:
La Jolla Institute for Immunology, San Diego, USA (Postdoctoral Fellow)
Immunology Center of Georgia, Augusta, USA (Asst. Research Scientist)
Year of Joining: 2025
Email: proy@iisc.ac.in
The fundamental objective of the lab is to employ a multidisciplinary approach to understand the triggers and mechanisms of immune cell reprogramming and its role in the pathophysiology of chronic inflammatory diseases in humans.
Highlights
- “What” are the key triggers that activate or reprogram immune cells in chronic diseases?
- “How” do reprogrammed immune cells contribute to inflammatory disease progression?
- “Which” rewired modules can be targeted for improved diagnosis, screening or therapy?
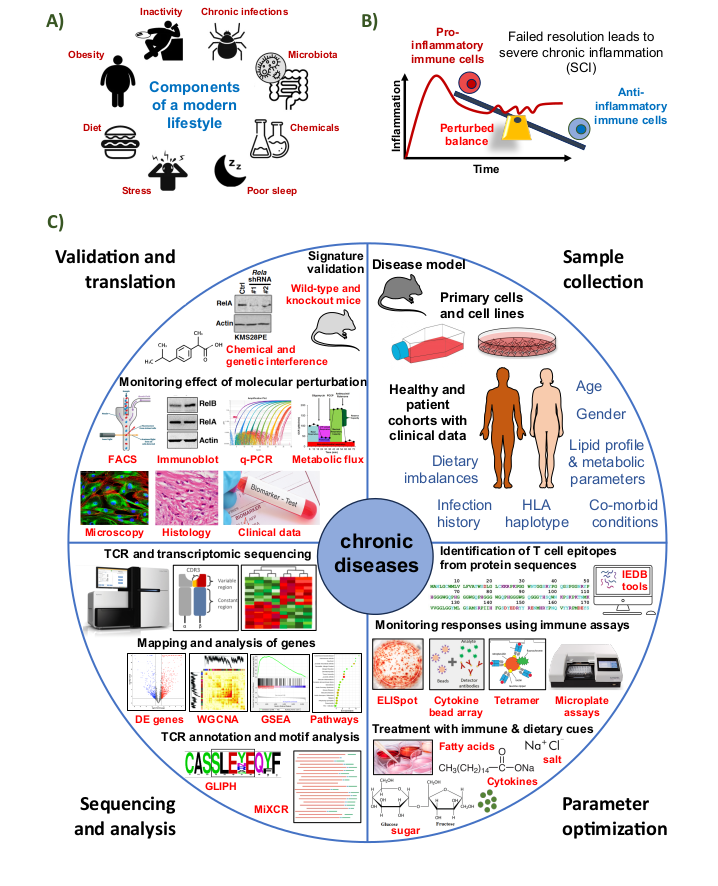
The immune system is a complex network of cells and organs that employ diverse specialized sensors and weapons to “recognize” and “eliminate” danger. This leads to a physiological state called “inflammation” that protects us from harmful invasion. Like every other war that history has ever witnessed, inflammation is associated with chaos, collateral damage and a loss of homeostatic balance. Under normal conditions multiple regulatory processes control the timing, strength, and duration of an inflammatory response. However, inappropriate control of inflammation can occur if immune cells escape the normal checkpoints and go rogue. Such a state of “corruption” is promoted by components of our modern lifestyle (panel A), resulting in an excess of pro-inflammatory “fighting” activity and reduction in anti-inflammatory “healing” activity (panel B). This unresolved inflammation is now implicated in the development of all major chronic diseases in humans, including cancer, metabolic diseases, neurodegenerative disorders, autoimmune conditions, cardiovascular and pulmonary diseases. Clinical diagnosis of chronic diseases is often delayed because their development involves a long asymptomatic latent phase. Traditional approaches are not sufficient for detecting early signs of immune dysfunction. Also, current treatments do not specifically mitigate the inflammatory risks. We aim to combine next-generation sequencing techniques, immunological and metabolic assays, computational analysis of high-throughput data, biochemistry, cellular and molecular biology, to understand immune cell maladaptation and identify precise biomarkers and druggable molecular targets (panel C).
Human blood is a valuable resource that can be accessed in healthy or diseased individuals. T cells can constitute up to 70% of Peripheral Blood Mononuclear Cells. Specific engagement of the T cell receptors (TCRs) with their cognate antigen triggers activation, polarization and formation of immunological memory. Different T cells express unique combination of molecules that is shaped by intrinsic and extrinsic factors. Circulating T cells can report to us about the status at distant tissue sites. Thus, gaining insights into “what they see and remember” (antigens and TCR), “where they go or reside” (tissue site and microenvironment), and “who they are” (phenotypes and programs) creates the possibility to track or modify disease-relevant human T cells. We have optimized pipelines to identify the antigenic targets, TCR specificity, phenotypic diversity and molecular signatures of antigen-specific T cells in the context of chronic metabolic diseases like cardiovascular disease (Roy, Nature Reviews Immunology, 2022; Roy, Circulation Research, 2022; Freuchet* and Roy*, Nature Immunology, 2023; Roy, Suthahar, Frontiers in Immunology, 2024). These will be combined with our expertise in utilizing pathway-based “reductionist” approaches (Roy, Oncogene, 2017; Chatterjee* and Roy* et al., Frontiers in Immunology, 2019) in gaining mechanistic insights into the perturbations of regulatory networks in pathogenic T cells.
Our goal will be to generate a “systems” level understanding of rewired immunological circuits.
Will be updated shortly
- Nettersheim FS et al. PD1 and CD73 on naïve CD4+ T cells synergistically limit responses to self. Nature Immunology. 2025 Jan; 26(1):105-115.
- Roy P et al. Identification of apolipoprotein B-reactive CDR3 motifs allow tracking of atherosclerosis-related memory CD4+T cells in multiple donors. Frontiers in Immunology. 2024. Mar 20:15:1302031.
- Freuchet A and Roy P et al. Identification of human exTregs as CD16+CD56+ cytotoxic CD4+ T cells. Nature Immunology. 2023 Oct; 24(10):1748-1761.
- Roy P et al. Immunodominant MHC-II (Major Histocompatibility Complex II) Restricted Epitopes in Human Apolipoprotein B. Circulation Research. 2022 Jul 22; 131(3):258-276.
- Roy P et al. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nature Reviews Immunology. 2022 Apr; 22(4): 251-265.
- Roy P et al. Non-canonical NFkB mutations reinforce pro-survival TNF response in multiple myeloma through an autoregulatory RelB:p50 NFkB pathway. Oncogene. 2017 Mar; 36 (10): 1417-1429.
Openings
Coming soon.